Have you ever heard someone say, “This soap is gentle on your skin because it has the right pH”? Or maybe your science teacher once dipped a little strip of paper into lemon juice and it turned bright red? That little moment was pH at work.
pH might sound like a big, complicated word, but it’s actually something you already see every single day. It decides whether something tastes sour, feels slippery, burns your tongue, or even keeps your plants alive. From the water you drink to the food you eat, pH is quietly shaping your world.
In this article, we’ll take pH apart piece by piece. We’ll look at what it really means, why it’s important, and how it touches every part of your life. Don’t worry—I’ll explain it in the simplest way possible, as if we’re sitting together at a table with a glass of lemonade and a little strip of pH paper. By the end, you’ll not only understand pH but also see the world around you in a whole new way.
So, let’s start with the big question: what exactly is pH?
What Does pH Really Mean?
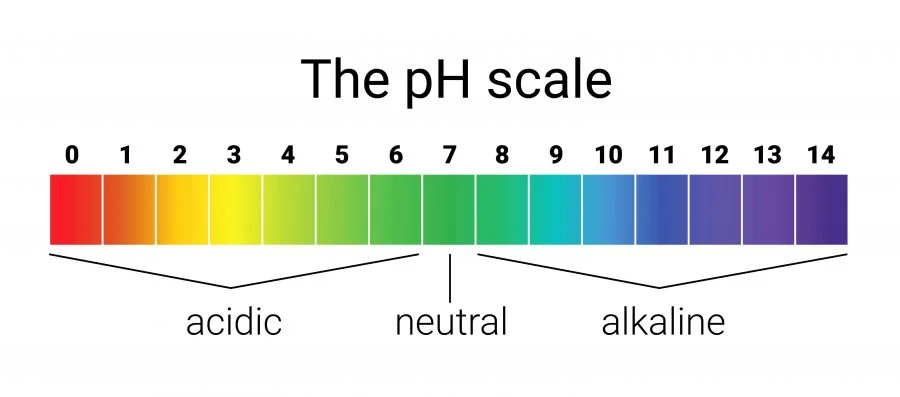
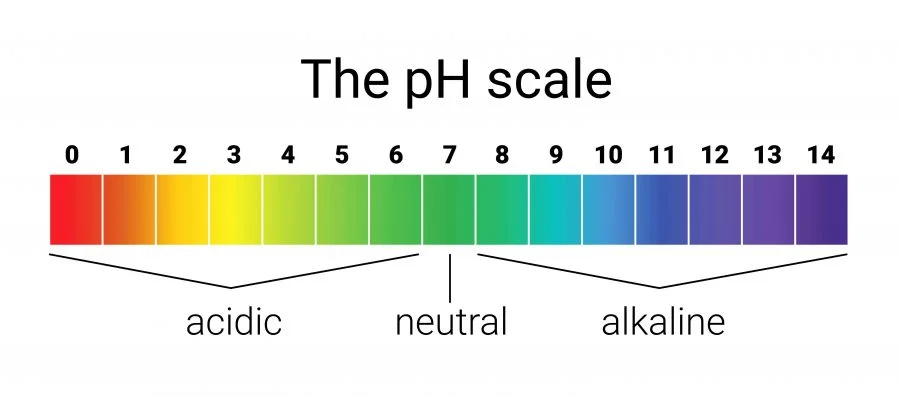
The word pH might look confusing at first, but it’s simply a way to measure how acidic or basic something is. Think of it as a scale that helps us understand how “sour” or how “soapy” a liquid feels.
Imagine a number line from 0 to 14. That’s the pH scale.
- If something is closer to 0, it’s very acidic. Acids are things that taste sour or can even burn.
- If something is closer to 14, it’s very basic (alkaline). Basics are things that feel slippery, like soap.
- Right in the middle, at 7, is neutral. That’s pure water—neither acidic nor basic.
So pH is like a ruler for liquids. It tells us where something falls between sour and soapy.
Everyday Examples of pH
Let’s make this more real. Here are some things you know and their pH levels:
- Lemon juice → around pH 2 (very acidic, which is why it tastes so sour).
- Tomatoes → around pH 4 (a little acidic, which is why they taste tangy).
- Pure water → pH 7 (neutral, no sourness or soapiness).
- Milk → pH 6.5 (just a little acidic, which is why it tastes mild).
- Soap → around pH 10 (basic, which is why it feels slippery on your skin).
- Bleach → pH 12 or higher (very basic and dangerous if touched).
See how different things in your kitchen already live on this scale? That’s pH at work.
Why Does Acid Taste Sour?
Here’s a fun fact: the reason your tongue knows something is acidic is because of the hydrogen ions in it. Don’t worry about the big word—just think of them as tiny “sour sparks.” The more sour sparks a liquid has, the lower its pH number, and the more sour it tastes.
That’s why lemon juice makes your lips pucker, while soap doesn’t. Soap doesn’t have those sour sparks—it has the opposite kind, which makes it slippery instead.
Why Do Basics Feel Slippery?
Have you ever noticed how soap feels a bit slimy between your fingers? That’s because basics (like soap) break down oils and fats. When they touch your skin, they start breaking down the natural oils there. That slipperiness is pH in action!
Why 7 Is “Just Right”
The middle of the pH scale—7—is special because it’s balanced. Water at pH 7 is neutral, which is why it’s safe to drink and doesn’t sting your tongue. Many living things, including us, need that balance to stay healthy. Too acidic or too basic, and things start going wrong.
So now we know: pH is a scale from 0 to 14 that shows if something is acidic, neutral, or basic. It’s all around us, from the food in your fridge to the soap in your bathroom.
Why Does pH Matter in Our Bodies?
Your body is like a carefully balanced machine. One of the ways it stays balanced is by keeping the right pH in different parts.
The pH of Your Blood
Your blood has a very tight pH range: around 7.35 to 7.45. That’s just slightly basic. If it goes even a little outside that range, your body can get very sick.
Your body works hard to keep blood in this safe zone. Your lungs and kidneys are like pH guardians. They remove extra acids or bases so the balance stays steady.
Stomach Acid
On the other hand, your stomach is very acidic—around pH 2. That’s even stronger than lemon juice! Why so acidic? Because your stomach needs to break down food and kill harmful bacteria.
If your stomach weren’t acidic, you wouldn’t be able to digest properly. But sometimes, too much acid causes heartburn. That’s when people take antacids—basic tablets that raise the pH just a little to calm the burning.
Saliva and Skin
Even your saliva has a pH—usually around 6 to 7. It helps keep your mouth healthy and fights germs.
Your skin, too, is slightly acidic. This “acid mantle” protects against harmful bacteria. That’s why soaps and creams often say “pH balanced”—they don’t want to upset your skin’s natural shield.
Why Does pH Matter in Food?
Every time you eat or drink, you’re dealing with pH.
- Fruits like oranges, strawberries, and grapes taste tangy because they are acidic.
- Bread rises because yeast makes acids during fermentation, changing the dough’s pH.
- Milk turns into yogurt when bacteria make it more acidic.
- Pickles are preserved in vinegar, which is acidic enough to stop harmful germs.
If food has the wrong pH, it can spoil quickly or taste strange. That’s why bakers, chefs, and food scientists often check pH carefully.
Why Does pH Matter in Nature?

The environment also depends on pH.
Soil pH and Plants
Plants can only grow well if the soil has the right pH. Some like more acidic soil (like blueberries), while others need neutral soil. If the soil is too acidic or too basic, plants can’t absorb nutrients properly. That’s why farmers test soil pH before planting.
Water pH and Animals
Fish and other animals in lakes and rivers need water with the right pH. If the water becomes too acidic (like from acid rain), fish may die. Even small changes in water pH can harm whole ecosystems.
Ocean pH
Our oceans are slowly becoming more acidic because of extra carbon dioxide in the air. This change is dangerous for coral reefs and sea creatures that rely on balanced pH to build their shells.
So now you see—pH matters because it keeps our bodies healthy, keeps food safe and tasty, and keeps nature in balance. Without the right pH, life as we know it would not survive.
How Do We Measure pH?
We know pH is a scale from 0 to 14, but how do we actually check where something falls on that scale? Luckily, scientists (and even students at home) have simple tools for this.
pH Paper (Litmus Paper or Indicator Strips)
The easiest way to test pH is with a little strip of paper. You dip it into a liquid, and the paper changes color. Then, you match the color to a chart.
- If it turns red or orange, the liquid is acidic.
- If it turns green, it’s neutral.
- If it turns blue or purple, it’s basic.
Litmus paper is simple—it only tells you if something is acidic or basic. But full pH strips are more detailed. They give you the exact number, like 3, 6, or 11.
This tool is great for quick experiments in the kitchen or classroom.
pH Meters
In labs and professional work, people use pH meters. These are electronic devices with a small probe you dip into a liquid. The meter shows the pH on a screen.
Meters are very accurate, but they need care—you have to clean them, store them in solution, and sometimes reset (calibrate) them. They’re a bit too fancy for most kids, but science classes sometimes use them.
Natural Indicators – Using Plants!
Here’s the fun part: you don’t always need special paper or machines to see pH. Nature already gives us indicators—plants that change color depending on acidity.
Red cabbage is the best example. It has a pigment called anthocyanin. This pigment changes color when the pH changes:
- Acidic liquids turn it red or pink.
- Neutral liquids turn it purple.
- Basic liquids turn it blue or green.
That means you can boil cabbage leaves in water to make purple juice, and then use that juice as your very own homemade pH test.
Safe, Fun pH Experiments for Kids

Now let’s turn your kitchen into a mini science lab. These experiments are safe and fun, but always do them with an adult nearby.
1. Lemon Juice vs. Baking Soda Water
- Pour some lemon juice into a cup.
- Pour baking soda mixed with water into another.
- Dip pH paper into both.
You’ll see lemon juice is acidic, and baking soda water is basic. This simple test shows how everyday ingredients fall on the pH scale.
2. Cabbage Indicator Magic
- Boil chopped red cabbage in water for 10 minutes.
- Strain out the leaves. You’ll have purple liquid—your indicator.
- Pour a little into three glasses.
- Add lemon juice to one (it turns pink).
- Add baking soda to another (it turns green).
- Leave one plain as your control (it stays purple).
Kids love this because it feels like a magic trick. But really, it’s chemistry in action.
3. Testing Everyday Liquids
Gather safe liquids from around your home: orange juice, vinegar, milk, cola, soapy water. Test each one with pH paper or cabbage juice.
Make a chart of results. Compare which are acidic, which are basic, and which are close to neutral.
This simple game turns your fridge into a science experiment.
By measuring pH, kids see science right in front of them—changing colors, bubbles, and surprising results. Suddenly, “pH” isn’t a scary word from a textbook. It’s something they can touch, test, and understand.
How pH Connects to Real-World Problems

pH is not just for classrooms and experiments—it’s everywhere around us. When it goes out of balance, big problems can happen. Let’s look at some examples.
pH and Pollution
Acid Rain.
When factories and cars release gases like sulfur dioxide and nitrogen oxides, these gases mix with water in the air. The result is acid rain—rain with a pH lower than normal.
Normal rain is slightly acidic, around pH 5.5. Acid rain can drop much lower. That may not sound like a big change, but it damages forests, makes lakes too acidic for fish, and even wears away stone buildings and statues.
Rivers and Lakes.
Water animals are very sensitive to pH. A lake that changes from neutral to slightly acidic can lose many types of fish and plants. Scientists regularly test the pH of rivers and lakes to keep an eye on water quality.
pH and Farming
Plants depend on the right soil pH to grow. If soil is too acidic, plants cannot take in important nutrients like calcium and magnesium. If it’s too basic, they may miss out on iron or phosphorus.
Farmers test soil pH before planting. If it’s too acidic, they add lime (a basic material) to raise the pH. If it’s too basic, they may add sulfur or compost to bring it down.
This shows how pH directly controls food supply. Without balanced soil, crops don’t grow well, and harvests shrink.
pH and Human Health
Stomach Acid.
As we learned earlier, your stomach needs strong acid that helps in digestion of food. But too much acid causes heartburn. That’s why antacids help—they are bases that bring the pH back toward balance.
Blood pH.
Your blood pH is tightly controlled. If it drifts too far, it signals serious health problems. Doctors sometimes test blood pH to check how well your lungs and kidneys are working.
Skin and Hair Care.
Your skin has a natural protective layer that is slightly acidic. Many shampoos and soaps advertise “pH balanced” because if they are too harsh, they can damage this layer and cause dryness or irritation.
pH in Swimming Pools
Have you ever swum in a pool and felt your eyes sting? That’s often because the pH of the water is not balanced.
- If pool water is too acidic, it irritates skin and eyes and can even damage the pool itself.
- If it’s too basic, chlorine doesn’t work properly, and bacteria can grow.
That’s why pool owners check pH regularly and add chemicals to keep it close to neutral, usually around pH 7.2 to 7.8.
pH and the Ocean
One of the biggest challenges facing the world today is ocean acidification. When carbon dioxide from the air dissolves into seawater, it lowers the ocean’s pH slightly. This small change makes it harder for sea animals like clams, oysters, and corals to build their shells. Over time, entire ecosystems could collapse if the pH keeps dropping.
Scientists call this a “quiet crisis” because it’s slow and hard to see, but it’s happening all over the world.
So you see, pH is not just a science fact—it’s a life fact. From the air we breathe to the food we eat and the water we drink, pH has the power to keep everything in balance—or push it out of balance.
Common Myths About pH

Because pH is linked to health, food, and the environment, people sometimes spread confusing or even wrong ideas about it. Let’s look at a few myths and set them straight.
Myth 1: “Drinking alkaline water will fix your body’s pH.”
You may have seen bottles of “alkaline water” at the store. They say drinking it will make you healthier by balancing your body’s pH.
Here’s the truth: your body already balances its own pH very carefully. Your blood stays in a tiny range (around 7.35–7.45). If it goes too far up or down, your lungs and kidneys step in right away to bring it back.
So even if you drink alkaline water, your stomach acid (pH 2) quickly makes it acidic again. The water doesn’t really change your blood pH at all.
That doesn’t mean alkaline water is bad—it’s just water with a slightly higher pH—but it’s not magic medicine.
Myth 2: “Acidic foods are dangerous.”
Some people think acidic foods like oranges, tomatoes, or vinegar are harmful because they have low pH. But your stomach acid is way stronger than any food you eat!
In fact, many acidic foods are good for you. They contain vitamins, help with digestion, and add flavor. The key is balance—too much of anything is never healthy, but acidity itself is not the enemy.
Myth 3: “You can change your whole body’s pH with food.”
There’s a popular idea called the alkaline diet, which claims that eating certain foods can make your blood less acidic and protect you from disease.
It sounds nice, but science shows it’s not really true. Again, your body controls your blood pH on its own. You can’t change it much just by eating spinach or drinking lemon water.
However, the diet does encourage people to eat more fruits and vegetables, which is healthy. So even if the reason is wrong, the outcome (more veggies, less junk food) is good.
Myth 4: “If something tastes sour, it must be dangerous.”
Not at all! Sour taste just means there are acids present. Some are harmful (like battery acid), but many are perfectly safe (like lemon juice or yogurt). The key is knowing the difference. That’s where pH testing and common sense come in.
Myth 5: “Basic (alkaline) cleaners are always safe.”
We often think “acid = bad” and “base = safe,” but bases can be dangerous too. For example, bleach and drain cleaner are very basic (pH 12–14) and can burn skin.
So whether something is acidic or basic, both extremes can be harmful. Neutral or near-neutral substances are usually the safest.
The Real Lesson for Kids
Here’s the simple truth:
- Your body already knows how to control its own pH.
- Food and water don’t change your blood pH much, but they do affect your overall health in other ways.
- Both acids and bases can be safe or dangerous, depending on what they are.
- pH is not about fear—it’s about understanding balance.
By clearing up these myths, kids can see pH as a helpful tool for learning and problem-solving, not something to be scared or misled about.
Why Learning About pH Helps Kids Grow
At first, pH may look like just another science topic. But when kids explore it hands-on, it becomes a powerful way to build life skills. Let’s look at how.
pH Builds Critical Thinking
When kids test the pH of everyday items, they learn to ask questions:
- Why did the paper turn red in lemon juice?
- Why did baking soda water turn green?
- Why does milk spoil faster than vinegar?
These aren’t just “science questions”—they’re thinking questions. Kids learn not to accept things at face value but to look for reasons and patterns. That habit—asking “why?”—is the foundation of all smart problem-solving.
pH Builds Patience and Focus
Testing pH isn’t instant. Kids may need to carefully dip paper strips, wait for color changes, or compare results with charts. They also see that experiments don’t always work the first time. Maybe they add too much juice, or the cabbage color looks unclear.
This teaches patience. It teaches focus. It reminds them that mistakes are part of learning, not the end of it.
At Debsie, we value this kind of steady learning. Just like pH experiments, our classes guide kids step by step, encouraging them to slow down, notice details, and keep trying until they succeed.
pH Teaches Problem-Solving
Imagine a child tests orange juice and soap water. The strip shows orange for the juice (acidic) and blue for the soap (basic). Now they wonder: what happens if I mix them?
This is problem-solving in action. They predict, test, and compare results. If the mixture turns closer to green, they’ve just learned that acids and bases can neutralize each other.
That’s chemistry, yes—but it’s also the same problem-solving process they’ll use in math, coding, or real-life decisions.
pH Sparks Curiosity
The best part about pH is how visible it is. Colors change, bubbles form, flavors shift. Kids can see and feel science happening. That spark of curiosity—“Why did it do that?”—is what turns a boring subject into an adventure.
When kids feel curious, they don’t just memorize facts. They want to explore, experiment, and learn more. At Debsie, that’s exactly what we encourage: not just learning answers, but learning how to discover answers on their own.
pH Builds Confidence
Finally, when a child successfully tests a liquid and explains it, they feel proud. That pride matters. It says: I can do science. I can figure things out. I can make sense of the world.
That confidence doesn’t stay in science class—it shows up everywhere. In math homework, in art projects, even in sports. Confidence is a seed, and pH experiments are one way to water it.
So, learning about pH isn’t just about acids and bases. It’s about building thinkers, problem-solvers, and confident explorers—the exact kind of kids Debsie aims to nurture every day.
Conclusion – pH: The Hidden Balance of Life
pH might sound like just another science term, but now you know it’s much more than that. It’s the quiet balance that keeps your body healthy, shapes the taste of your food, and protects life in our rivers, soils, and oceans. From the sourness of lemon juice to the slipperiness of soap, pH is working all around you—even when you don’t see it.
Understanding pH also helps kids build something bigger than just knowledge. It teaches them to notice details, ask smart questions, solve problems, and stay curious. It shows them that science is not locked in textbooks—it’s in their glass of water, their garden soil, even the swimming pool on a hot day.
At Debsie, we believe every subject can feel this alive. Just like pH makes science visible, our classes make learning exciting and real. Kids don’t just learn facts—they experiment, explore, and grow the skills they’ll use for life: focus, patience, smart thinking, and confidence.
So the next time you sip lemonade, wash your hands with soap, or swim in a pool, remember: you’re not just experiencing daily life—you’re experiencing pH in action.
👉 Ready to turn everyday curiosity into powerful learning? Sign up for a free trial class with Debsie today and watch your child rise—just like their confidence, their curiosity, and their love for learning.
Read Next: