Think about a bowl of fruit salad. You can see the apple slices, grapes, and bananas sitting together. Now think about a cookie. You can’t separate the sugar, flour, and butter once it’s baked—they’ve combined into something new.
This is the difference between a mixture and a compound. One is just things sitting together, and the other is things joining to form something completely new.
At first, the two might sound confusing. After all, both involve putting different substances together. But once you understand the key differences, you’ll never mix them up again. In fact, this simple idea can help you make sense of the food you eat, the air you breathe, and even the chemistry happening inside your body.
In this article, we’ll break it down step by step, using very simple words and clear examples you see every day. By the end, you’ll be able to spot mixtures and compounds everywhere around you—and explain the difference with total confidence.
What Is a Mixture?
A mixture happens when you take two or more substances and put them together, but they don’t actually change into something new. They simply sit together in the same space. Each substance keeps its own properties.
Think about this: if you mix a handful of rice with some beans, you now have a bowl of rice and beans. Nothing magical has happened. The rice is still rice. The beans are still beans. If you wanted to, you could separate them again just by picking them out.
That’s the secret of mixtures—they’re combinations, but not chemical transformations.
Why Mixtures Are Special
At first, mixtures may sound boring—just “stuff put together.” But mixtures are actually fascinating because they make up most of what we see around us. In fact, very few things in nature are pure substances. Almost everything is a mixture of something else.
The air you breathe? A mixture. The juice you drink? A mixture. The soil under your feet? A mixture. Even your blood flowing through your body is a mixture. Once you understand mixtures, you’ll start noticing them everywhere.
Two Main Types of Mixtures

Even though mixtures come in many forms, scientists usually divide them into two broad categories. Don’t worry—we’ll keep it simple.
1. Homogeneous Mixtures – Evenly Spread
“Homogeneous” sounds like a big word, but it just means same throughout. In a homogeneous mixture, you can’t see the different parts, because everything is spread out evenly.
For example:
- Salt water. Once salt dissolves, the liquid looks clear. You can’t see the salt crystals anymore, but they’re still there. If you boil the water, the salt will reappear.
- Air. It looks invisible, but it’s actually a smooth blend of nitrogen, oxygen, carbon dioxide, and tiny amounts of other gases. You don’t see the layers—it’s mixed evenly.
- Sugar in tea. When you stir sugar into your tea, it vanishes into the liquid. You can’t see the sugar, but it’s mixed evenly with the water.
So, homogeneous mixtures are sneaky—they look like one thing, but they’re actually many things blended together.
2. Heterogeneous Mixtures – Unevenly Spread
“Heterogeneous” means different throughout. In these mixtures, the parts are not evenly mixed, and you can usually see them separately.
For example:
- Fruit salad. You can see the apples, grapes, and bananas all sitting together. Each fruit is separate.
- Cereal with milk. The flakes and milk don’t mix completely. They stay as two distinct parts.
- Sand and pebbles. When you scoop up sand with small rocks, you can clearly tell one from the other.
These mixtures are easier to spot because the different pieces are visible.
Everyday Life Is Full of Mixtures
Let’s pause for a moment. Look around you. You are literally surrounded by mixtures:
- Your backyard soil is a mixture of tiny minerals, sand, clay, dead leaves, and even tiny insects.
- Your blood is a mixture of plasma, red blood cells, white blood cells, and platelets.
- The milk in your glass is a mixture of water, fats, proteins, and vitamins.
- Even a simple sandwich is a mixture of bread, cheese, and whatever filling you put inside.
Everywhere you look, mixtures are quietly shaping your life.
How to Know If Something Is a Mixture
Here’s a quick test you can use to tell if something is a mixture: Can I separate the parts without changing what they are?
- If you can separate the parts by simple methods—like filtering, boiling, picking out, or using a magnet—then it’s a mixture.
- For example:
- Salt water → boil away the water, and the salt is left.
- Rice and beans → pick them apart with your fingers.
- Iron filings and sand → use a magnet to pull the iron out.
If the answer is yes, then you are looking at a mixture.
What Is a Compound?
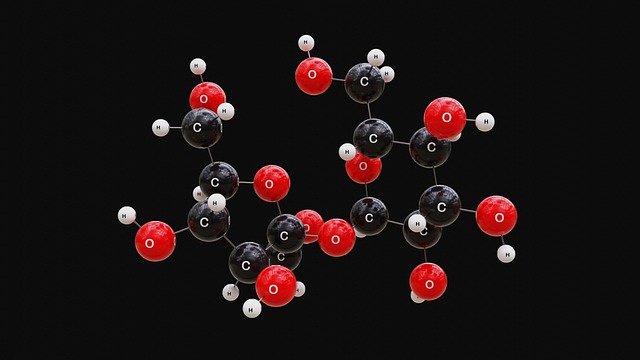
If a mixture is just substances sitting together, a compound is the opposite. In a compound, two or more elements actually join together chemically to form something brand new.
Here’s the key: in a compound, the original substances lose their separate identities. They combine so tightly that you can’t tell them apart anymore, and you can’t separate them easily.
A Simple Example: Water
Let’s take water. We write it as H₂O.
- Each molecule of water has two hydrogen atoms and one oxygen atom joined together.
- Hydrogen on its own is a gas that burns easily. Oxygen on its own is a gas that helps things burn.
- But when they chemically bond, they become water—a liquid that puts out fire!
This shows how a compound creates something totally new.
Another Example: Salt
Table salt is another compound. Its chemical name is sodium chloride (NaCl).
- Sodium is a soft metal that reacts dangerously with water.
- Chlorine is a poisonous yellow-green gas.
- But when they bond, they form safe, edible salt that you sprinkle on your food.
Again, the compound is completely different from the elements that made it.
How Compounds Are Formed
Compounds form because atoms want stability. Atoms either share or transfer electrons to become more stable, and in doing so, they create chemical bonds.
There are two main kinds of chemical bonds:
- Ionic bonds: where atoms transfer electrons (like sodium and chlorine in salt).
- Covalent bonds: where atoms share electrons (like hydrogen and oxygen in water).
Don’t worry about the big words—what matters is that compounds involve atoms locking together in a way that can’t be undone by simple physical methods.
Why Compounds Are Different
Let’s compare to mixtures:
- In mixtures, substances keep their own properties. In compounds, new properties are formed.
- Mixtures can be separated by physical means (picking apart, filtering, boiling). Compounds need chemical reactions to be broken apart.
- Mixtures are like roommates living in the same apartment. Compounds are like a married couple—they’ve joined and formed something new.
Everyday Examples of Compounds
Compounds are everywhere in your daily life.
- Carbon dioxide (CO₂): You breathe it out, and plants use it for photosynthesis.
- Sugar (C₆H₁₂O₆): A sweet compound made of carbon, hydrogen, and oxygen.
- Baking soda (NaHCO₃): Used in baking to make cakes rise.
- Rust (Fe₂O₃): When iron reacts with oxygen, it becomes iron oxide—a new compound.
Each of these is not just a “mixture” of elements. They’re something new entirely.
The Big Idea
Compounds show us the power of chemistry. When atoms bond, they don’t just sit together like pieces in a salad—they transform into something new, often with properties you could never guess from the original elements.
Mixtures vs Compounds – The Key Differences

We’ve explored mixtures and compounds separately. Now, let’s bring them together and compare them directly so you can see how different they really are.
How They Form
A mixture forms when two or more substances are placed together without changing their basic nature. For example, if you stir sand into salt, both remain exactly as they are. You can still taste salt as salt and sand as sand. A compound, on the other hand, forms when substances combine chemically. The result is something entirely new. Hydrogen gas and oxygen gas are nothing alike, but when they bond chemically, they create water—something completely different from either of its parts.
Their Properties
In a mixture, each substance keeps its own characteristics. A fruit salad is the perfect example. The banana remains banana, the apple remains apple, and nothing about them has truly changed. A compound is very different because it gains new properties once the atoms bond. Sodium on its own is a soft metal that reacts violently with water. Chlorine is a poisonous yellow-green gas. Yet together, they make common table salt, which is safe to eat and completely unlike its dangerous ingredients.
How They Can Be Separated
Separating a mixture is usually simple, because the substances inside it are only physically combined. You can separate salt water by evaporating the water and leaving the salt behind. You can pull iron filings out of sand using a magnet. With a compound, the story is different. Compounds cannot be separated by physical means. To split water into hydrogen and oxygen, you need a chemical process called electrolysis. This shows that compounds are bonded at a much deeper level than mixtures.
The Role of Ratios
Another clear difference lies in the proportions of the ingredients. Mixtures can be made in any ratio. You can add a little sugar to your tea or a lot—it is still sugar in tea. Compounds, however, always follow strict formulas. Water is always two hydrogen atoms bonded to one oxygen atom. If you change that ratio to two hydrogens and two oxygens, you no longer have water—you now have hydrogen peroxide, a completely different compound.
Everyday Examples
When you look at your surroundings, mixtures are all around you. The air you breathe is a mixture of nitrogen, oxygen, and other gases blended together. Soil is another mixture, made up of sand, clay, minerals, and organic matter. Compounds, on the other hand, show up in forms like carbon dioxide in fizzy drinks, sugar in sweets, or rust on an old bicycle. These are not simple blends—they are substances with new properties created by chemical bonds.
The Big Analogy
If you want an easy way to keep the difference clear, think of a classroom and a cake. A classroom full of students is like a mixture. Each student remains an individual, even though they are sitting together in the same room. A cake is like a compound. Once you bake the flour, sugar, butter, and eggs, they no longer exist as separate ingredients. They have transformed into something entirely new that cannot be undone.
Why Mixtures vs Compounds Matter in Daily Life

At first, learning the difference between mixtures and compounds may feel like just another science lesson. But once you start noticing it in the real world, you’ll see how useful this knowledge really is. It explains so much of what you see, touch, and use every day.
Food and Cooking
Cooking is one of the best places to see mixtures and compounds at work. A salad is a mixture—you can still separate the lettuce, tomato, and cucumber. But when you bake bread, you are no longer looking at a mixture. The flour, water, and yeast react together and create new compounds like carbon dioxide, which makes the dough rise, and other substances that give bread its flavor.
Even the salt you sprinkle on food is a compound, while the juice in your glass is a mixture of water, sugar, and flavor. By knowing the difference, you can understand why some foods can be separated into their ingredients while others can never return to their original form.
Medicine and Health
The pills you take when you are sick often contain compounds. Aspirin, for example, is a chemical compound with a precise formula. You cannot separate it into its elements without breaking it chemically. On the other hand, a cough syrup may be a mixture of sugar, flavor, and medicine dissolved in water.
Doctors and pharmacists need to understand this difference because it affects how medicines are made, stored, and used. A mixture might be flexible in its proportions, but a compound must always be exact, otherwise it becomes a completely different substance.
The Environment Around Us
Look at the world outside, and you will see mixtures everywhere. Soil is a mixture that supports plant life. Air is a mixture of gases that allows humans and animals to breathe. These mixtures can change—sometimes more oxygen, sometimes more carbon dioxide—but they are still mixtures.
Compounds in nature, however, are fixed. Water is always H₂O. Carbon dioxide is always CO₂. Plants need both mixtures (like soil) and compounds (like water and carbon dioxide) to grow. Without understanding this, we wouldn’t know how ecosystems function.
Science and Technology
In industries and technology, mixtures and compounds play different roles. Gasoline in a car is a mixture of different hydrocarbons. Engineers can adjust that mixture to improve performance. But materials like plastics, metals, and ceramics are compounds with fixed chemical structures. You can’t just change their ratios without creating a completely new substance.
Even in space travel, this knowledge is critical. Rocket fuel is a carefully prepared mixture, while the oxygen that astronauts breathe is a compound. Both need to be understood clearly for safety.
Why It Really Matters
Understanding mixtures versus compounds is not just about passing a science test. It’s about being able to explain everyday life. Why can you pick marshmallows out of cereal but not flour out of cake? Why can a doctor change the ingredients of a syrup but never change the formula of a pill? Why does soil vary from place to place, but water is always the same wherever you go?
The answers all come back to this simple distinction: mixtures can be separated, adjusted, and flexible. Compounds are fixed, precise, and form entirely new substances.
Fun Experiments with Mixtures and Compounds

Science becomes memorable when you can touch it, taste it, or see it change right before your eyes. Mixtures and compounds are perfect for this kind of learning because they are everywhere in daily life.
Experiment 1: Making and Separating a Mixture
Take a clear glass and add a spoonful of sand and a spoonful of salt. Stir them together. At first, it just looks like a pile of grains—but this is a mixture. Both the sand and the salt are still there, unchanged.
Now try to separate them. If you pour water into the glass, the salt dissolves, but the sand does not. You can then filter the sand out with a piece of cloth or paper. Later, if you let the salty water evaporate, the salt crystals will reappear.
This shows that mixtures can be separated by physical means because the substances never lost their identity.
Experiment 2: Watching a Compound Form
Now let’s see what happens when a compound is created. Mix a spoonful of baking soda with a splash of vinegar in a cup. Immediately, bubbles of gas appear and the liquid fizzes. What’s happening?
The baking soda and vinegar are not just sitting together like a salad—they are reacting. They form new substances, one of which is carbon dioxide gas. That’s why you see bubbles. This is the birth of a compound and a sign that chemical change has taken place.
Unlike a mixture, you cannot separate the baking soda and vinegar back into their original form once they’ve reacted. The transformation is permanent.
Experiment 3: The Mystery of Sugar vs. Salt in Water
Take two glasses of warm water. Stir sugar into one and salt into the other until both disappear as they get dissolved in the water. To the eye, they look the same. But here’s the twist: sugar in water is a mixture, while salt in water is technically forming a solution that involves ions breaking apart.
Later, if you let the water evaporate from both glasses, the sugar and salt will return. This shows again how mixtures and certain dissolving processes allow recovery of original substances, unlike compounds where elements are chemically locked together.
Experiment 4: Cooking as Chemistry
Cooking is one of the easiest ways to show the difference. Crack an egg into a pan. The egg white is a mixture of water and proteins at first. But when you heat it, the proteins bond permanently and form a solid. You cannot turn the cooked egg white back into its raw form. That transformation is chemical—it behaves like a compound reaction.
In contrast, when you mix a bowl of flour, sugar, and chocolate chips, it remains a mixture. You can still pick the chips out if you want. But once you bake those ingredients into a cookie, they change into something entirely new, and you cannot reverse it.
What Kids Learn from These Experiments
Through these simple activities, kids see that mixtures are flexible and reversible, while compounds are fixed and transformative. More importantly, they learn that science isn’t just in textbooks—it’s in their kitchen, their classroom, and their everyday world.
At Debsie, this is exactly how we teach. We let children try, fail, test again, and discover the truth with their own hands. That way, knowledge doesn’t just stay in their memory—it becomes part of their experience.
Life Lessons from Mixtures and Compounds
At first glance, mixtures and compounds may seem like small chemistry topics. But when kids explore them deeply, they discover patterns and lessons that go far beyond the science classroom. These lessons help shape how they think, how they solve problems, and how they see the world.
Mixtures Teach Flexibility
Mixtures remind us that sometimes, things can come together without losing their uniqueness. In a salad, each ingredient keeps its own flavor. In the air, each gas keeps its own identity.
For kids, this shows that not everything has to blend completely to work well. Just like friends in a group, everyone can keep their individuality but still form something bigger together. It’s a quiet but powerful lesson about diversity, teamwork, and respecting differences.
Compounds Teach Transformation
Compounds tell a different story. When elements come together chemically, they change completely. Hydrogen and oxygen form water. Sodium and chlorine form salt. These are transformations that cannot be undone.
For children, this is a lesson in commitment and growth. Sometimes, change is permanent—and that’s not bad. It’s how progress happens. Just as elements change to form new substances, people grow and transform as they learn new skills or overcome challenges.
Both Teach Patience
Whether it’s separating a mixture by evaporation or watching a chemical reaction fizz, both concepts show kids that science takes time. A child waiting for water to evaporate to recover salt is also learning patience. A child watching a cookie bake in the oven is learning that transformation cannot be rushed.
Patience is a skill every learner needs, and science provides it naturally.
Both Teach Problem-Solving
Separating a mixture is a puzzle. Should I filter it, use a magnet, or evaporate the liquid? Creating a compound is another kind of puzzle—what happens if I mix this with that?
In both cases, kids learn to think step by step. They form hypotheses, test them, and adjust when things don’t work out. This is the same kind of problem-solving they’ll use in math, coding, and even everyday decisions.
Both Spark Curiosity
Mixtures and compounds are also wonderful for sparking curiosity. Kids start to ask:
- Why can I separate this mixture but not that one?
- Why does this reaction create bubbles?
- What would happen if I added more salt, or more vinegar?
That curiosity is the fuel of lifelong learning. When nurtured, it leads kids not only into science but also into exploring history, technology, and the arts with the same excitement.
The Debsie Approach
At Debsie, we don’t just tell kids: “Here’s the difference between mixtures and compounds.” We let them explore it hands-on. We encourage them to notice, test, and question. And in the process, they pick up more than science—they build confidence, focus, resilience, and the joy of discovery.
For us, mixtures and compounds aren’t just chemistry terms. They are doorways into teaching kids how to see patterns, embrace change, and trust their own ability to figure things out.
Conclusion – Mixtures vs Compounds
Mixtures and compounds may seem like small topics, but they are at the heart of chemistry. A mixture is when substances come together but stay themselves, like sand in water or fruits in a salad. A compound is when elements bond and transform into something completely new, like hydrogen and oxygen becoming water. This simple difference explains much of the world around us—from the food we cook to the medicines we use.
For kids, learning about mixtures and compounds is more than memorizing definitions. It’s about discovering patterns, asking questions, and understanding why things behave the way they do. Each experiment, whether it’s separating salt from water or watching vinegar fizz with baking soda, teaches patience, problem-solving, and curiosity. These lessons go far beyond science—they shape how children think and approach challenges in life.
At Debsie, we bring these everyday wonders alive. Our expert teachers use simple, hands-on activities to turn abstract science into something children can see, touch, and truly understand. In the process, kids not only learn the difference between mixtures and compounds, but also gain confidence, focus, and a deeper love for learning.
👉 Give your child the gift of discovery. Book a free trial class at Debsie today.
Read Next: